Abstract
Background
Orthostatic hypotension due to autonomic dysfunction is a well-known complication of light chain (AL) amyloidosis, which can become progressively debilitating and difficult to manage. Treatment of the underlying plasma cell dyscrasia will eventually decrease further amyloid deposition. Management of orthostatic hypotension secondary to AL amyloidosis improves quality of life and facilitates delivery of plasma cell therapy. Pharmacologic interventions include fludrocortisone, sympathomimetic agents such as midodrine, droxidopa, the acetylcholinesterase inhibitor pyridostigmine or the norepinephrine transporter (NET) inhibitor atomoxetine. Fludrocortisone is often poorly tolerated in amyloid patients because it may exacerbate edema. Droxidopa is a synthetic amino acid analog that is directly metabolized to norepinephrine by dopa-decarboxylase, which increases blood pressure (BP) by inducing peripheral arterial and venous vasoconstriction.
Aims
To assess the effectiveness of droxidopa in patients with AL amyloidosis with severe orthostatic hypotension refractory to midodrine. Also, to describe effective dose of droxidopa, duration of therapy, adverse effects and reasons for discontinuation.
Methods
A regional retrospective study was done in patients with AL amyloidosis with severe, refractory orthostatic hypotension who received droxidopa. Retrospective data was reviewed from 2018 to 2021 at a single academic center in the United States.
Results
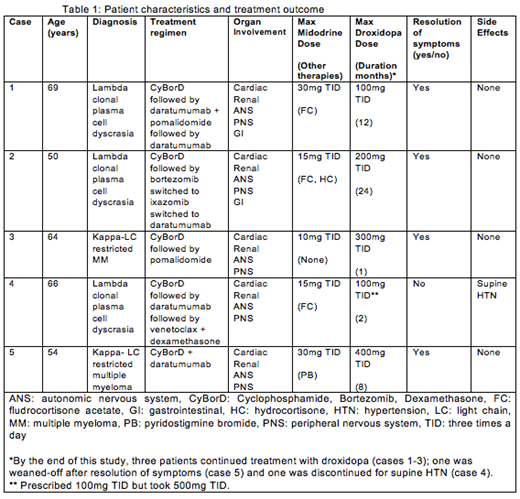
Five patients with AL amyloidosis were included in the study; three patients had lambda-restricted plasma cell dyscrasia and two had multiple myeloma (MM) associated AL amyloidosis (both kappa light chain restricted). Of the five patients, all had cardiac, renal, autonomic nervous system and peripheral nervous system involvement and two of the five had gastrointestinal involvement as well. Given their poor performance status and advanced organ involvement, none of the patients were eligible for high-dose intravenous melphalan with autologous peripheral blood stem cell transplantation (HDM/SCT), and thus were treated with cyclophosphamide, bortezomib and dexamethasone (CyBorD). All patients achieved very good partial response to complete hematologic response. The main findings are summarized in table 1.
All patients had severe, symptomatic orthostatic hypotension that was objectively defined as a decrease in systolic blood pressure by 20 millimeters of mercury (mmHg) or a decrease in diastolic blood pressure of 10 mmHg from supine to either sitting or standing in the clinic or at home (Freeman R consensus statement on the definition of orthostatic hypotension, 2011). Initial treatment for all patients included midodrine, ranging from 5 to 30mg TID based on individual tolerance. Three of the patients also were initially treated with fludrocortisone 0.05 to 0.2mg daily (use limited by fluid retention). Only one patient was on pyridostigmine 30mg TID (case 5). Given persistence of symptoms despite therapy, droxidopa was started at 100mg TID in all patients, and the dose was titrated as tolerated. None required the maximal approved dose of 600mg TID.
The indication to start droxidopa was based on refractory, symptomatic orthostatic hypotension in all five patients. After initiation of droxidopa, all except for one patient reported improvement both in symptoms of lightheadedness as well as measurements of orthostatic blood pressure values. By the end of this study, three patients continued treatment with droxidopa (cases 1-3); one was weaned-off after resolution of symptoms (case 5) and one was discontinued due to supine hypertension (case 4).
Conclusion
Data shows that droxidopa is an effective treatment of orthostatic hypotension refractory to midodrine in patients with AL amyloidosis. Slow titration may be important to minimize rapid changes in blood pressure. Further studies are warranted to assess droxidopa's safety and compare with other treatments for orthostatic hypotension.
Hughes: Amgen: Speakers Bureau; Rigel: Other: Advisory Board, Research Funding; Abbvie: Speakers Bureau; Karyopharm: Other: Advisory Board, Speakers Bureau. Sanchorawala: Celgene: Research Funding; Takeda: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Prothena: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees; Caelum: Membership on an entity's Board of Directors or advisory committees, Research Funding; Regeneron: Membership on an entity's Board of Directors or advisory committees; Proclara: Membership on an entity's Board of Directors or advisory committees; Oncopeptide: Research Funding; Karyopharm: Research Funding; Sorrento: Research Funding; Pfizer: Honoraria. Sloan: Nuvectis: Consultancy; Abbvie: Consultancy; Stemline: Consultancy; Astrazeneca: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; Pharmacosmos: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal